NON-AQUEOUS AND AQUEOUS ELECTRIC CONDUCTIVITY
Electric conductivity of aqueous solutions differs from electric conductivity of non-aqueous solutions by many orders of magnitude. Non-aqueous solutions include non-polar liquids such as toluene, hexane, dodecane when dielectric constant is very low. They are important for many novel products like electronic paper, batteries, and fuel cells. Consequently, dispersions prepared on their basis depend on ionic composition, which can be characterized with conductivity measurement. Therefore, utilization of this simple method requires a special conductivity probe capable of measuring conductivities of liquids over a very wide dynamic range, from 10-11 S/m up to 10-4 S/m.
There are two models that can conduct such measurement: standalone Non-aqueous conductivity meter DT-700 and Option OP 0041 for DT 1202. Precision of a single measurement is ±(1%+10E-11S/m) over the complete range and can be statistically improved for multiple measurements. This Figure demonstrate results of such conductivity measurements for several liquids with distinctively different conductivities:
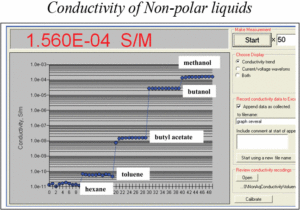
Application of this instrument for characterizing non-polar liquids and dispersions can be found in several published papers, see for example:
Evidently, one of the most interesting applications of this method is characterization of surfactant adsorption from non-polar liquids into porous materials. The following paper describes this application:
